Evaluation of Drug Efficacy–Quantitative Evaluation of Spheroid Size
The efficacy of anticancer drug 5-FU was evaluated through the quantification of the cross-sectional area of spheroids based on bright field image analysis with the system.
Objectives
Recently, 3D tumor spheroids have been considered to be useful as in vitro models of solid tumors. The change in size of 3D spheroids induced by the treatment of anticancer drugs is an indicator that is highly relevant to their ability to inhibit cell proliferation. The efficacy of anticancer drugs can be quantitatively and efficiently evaluated through bright field image analysis of the change in spheroid size using a microscope in combination with the system. In this study, the size changes of HT-29 3D spheroids induced by 5-FU were quantitatively evaluated.
Preparation of samples
A cell suspension of HT-29 was seeded into a PrimeSurface® 96U plate (SUMITOMO BAKELITE CO., LTD) at 750 cells/well to prepare spheroids. 5-FU was added to the 3D spheroids four days after the start of cell culture, and the culture was continued for another four days. Under the same conditions, HT-29 2D monolayers were prepared in a cell adhesive microplate followed by the addition of 5-FU to 2D monolayers .
Conclusion
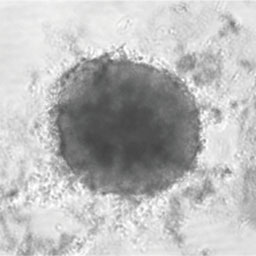
Acquisition and analysis of images of 3D-spheroids or 2D-monolayers composed of HT-29
Bright field images of microplates containing HT-29 3D spheroids were obtained with the system (Figure 1). Several images containing an equatorial plan were obtained for one spheroid, followed by mean intensity projection and statistical analysis at each concentration of 5-FU. The cross sectional area of HT-29 3D spheroids decreased with an increase in the concentration of 5-FU, which indicated that 5-FU inhibits cell proliferation in spheroids (Figure 2). For the HT-29 2D monolayer stained by Hoechst 33342, the adhesion area was calculated based on the fluorescent images obtained with the system. The adhesion area of HT-29 2D monolayers decreased with an increase in the concentration of 5-FU (Figure 2). These results indicated that the system enables evaluation of drug efficacy in 3D-spheroids and 2D- monolayers.

PrimeSurface is a registered trademark of Sumitomo Bakelite Co., Ltd.
Olympus is a registered trademark, and NoviSight and Insightful Analysis, Intelligent Answers are trademarks of Olympus Corporation.
Products Related to This Application
was successfully added to your bookmarks
Maximum Compare Limit of 5 Items
Please adjust your selection to be no more than 5 items to compare at once
Not Available in Your Country
Sorry, this page is not
available in your country.
