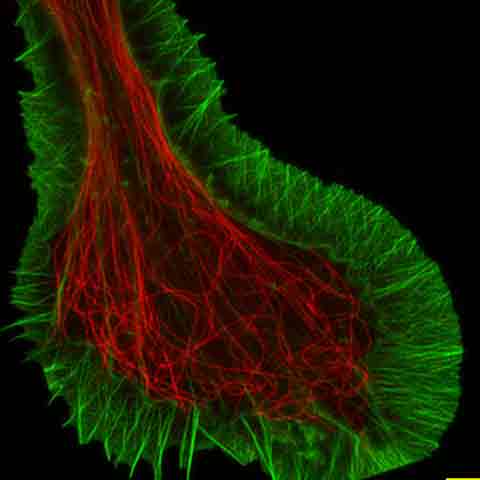
SUPER RESOLUTIONResolve confocal images down to 120 nm XY resolution using the confocal technique and Olympus super resolution (OSR). *Image: Stress fibers of Hela cell: Antibody staining with Phalloidin-Alexa488 (green) for actin, Alexa 568 (red) for myosin heavy chain. Image courtesy of: Keiju Kamijo,Ph.D. Division of Anatomy and Cell Biology, Faculty of Medicine, TOHOKU Medical and Pharmaceutical University |
|---|
FAST IMAGINGFast imaging using a spinning disk confocal and fast super resolution processing enable a live display of samples. The viability of cells during confocal time-lapse imaging is prolonged thanks to less phototoxicity and bleaching in 3D. *Movie: GFP-EB3 at the tip of elongating microtubules in HeLa cell.Image courtesy of: Dr.Kaoru Katoh , Biomedical Research Institute, National Institute of Advanced Industrial Sciences and Technology |
|---|
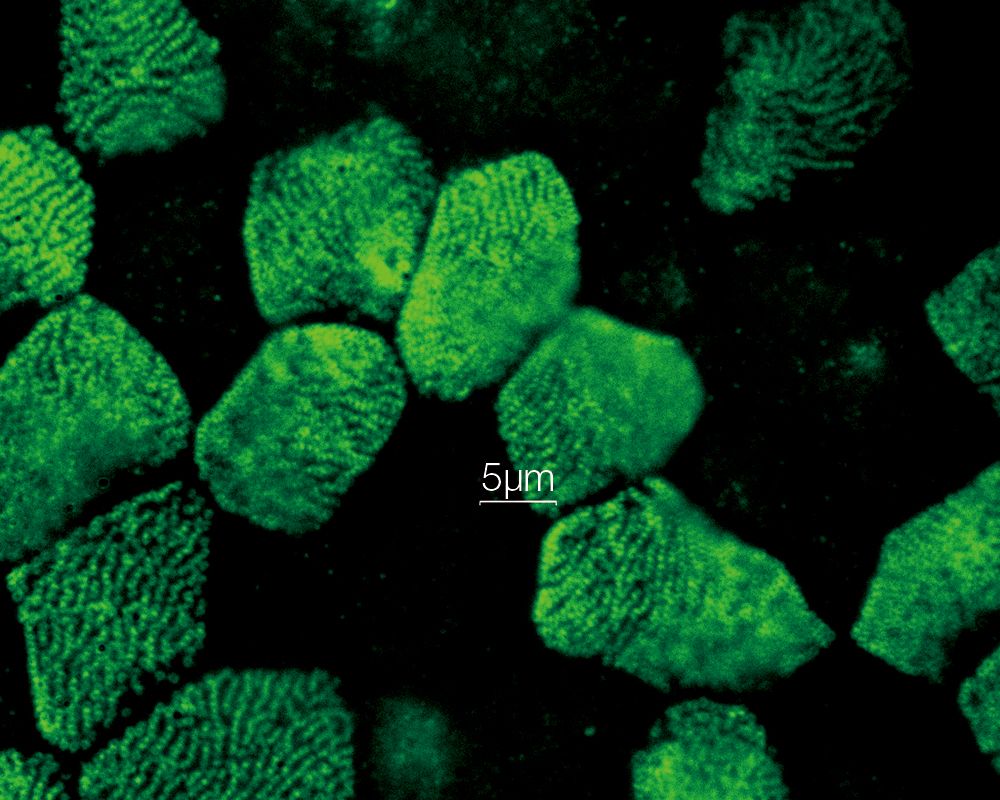
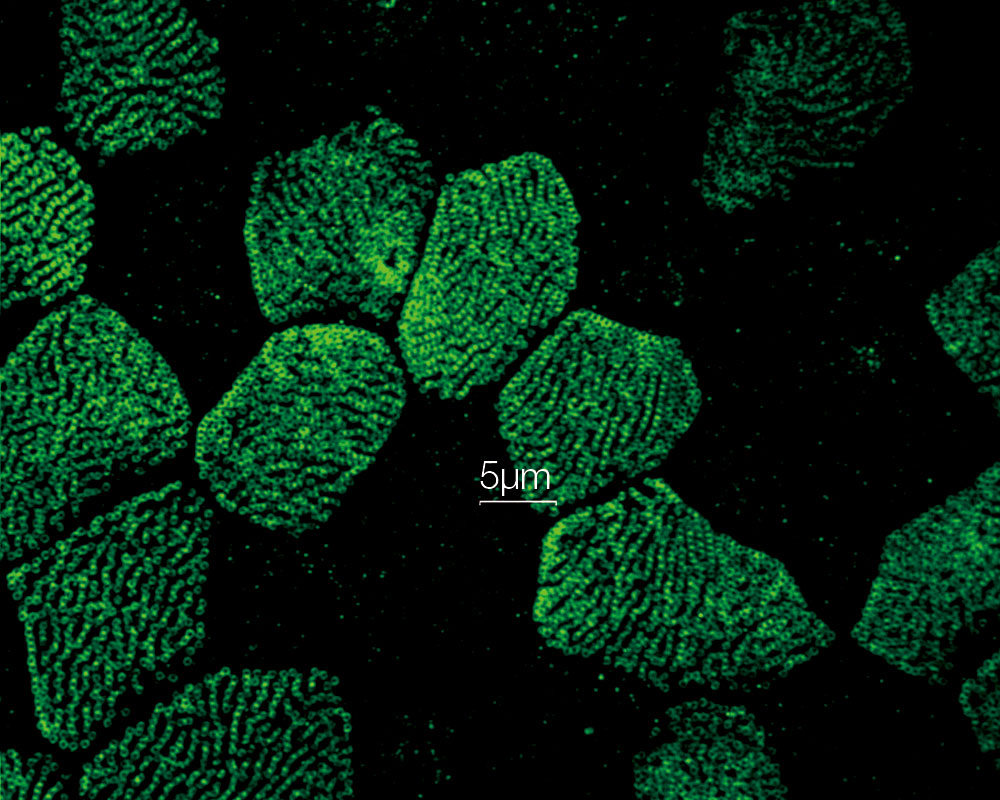
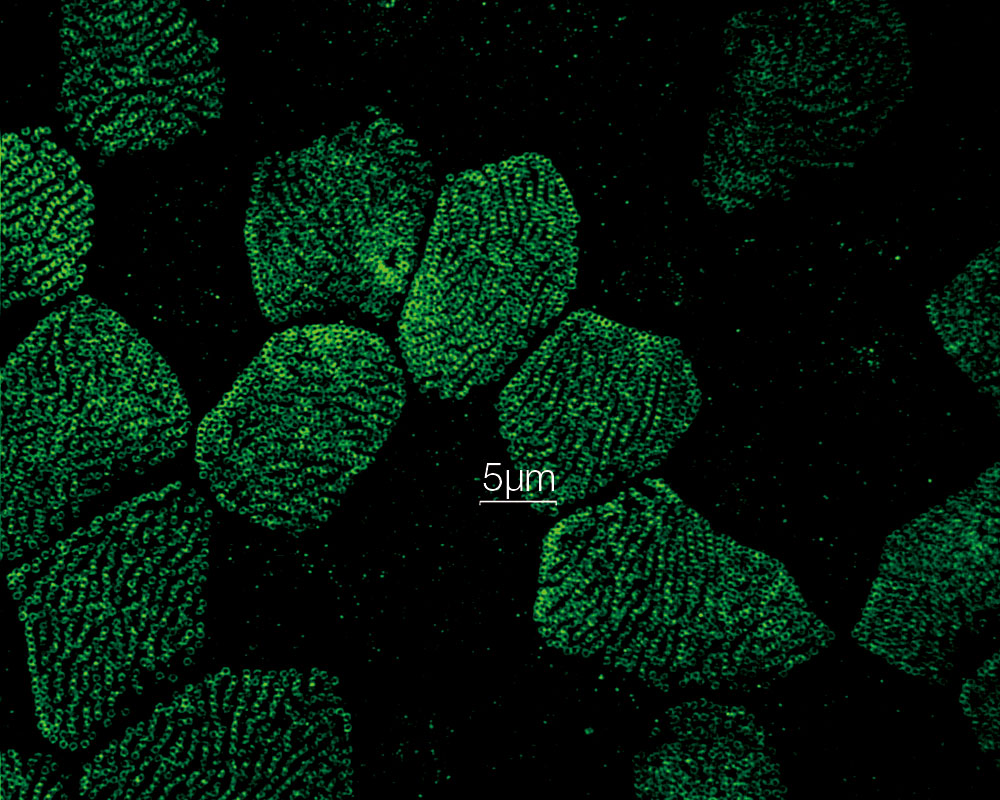
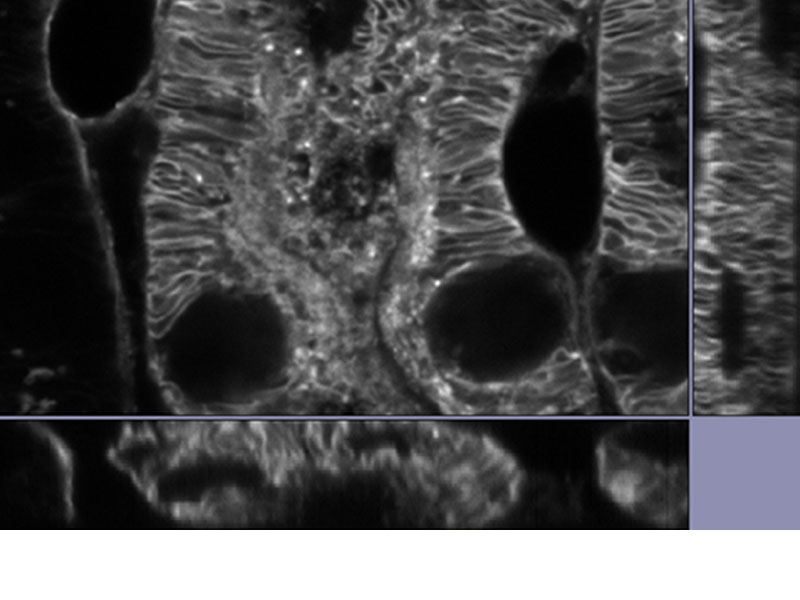
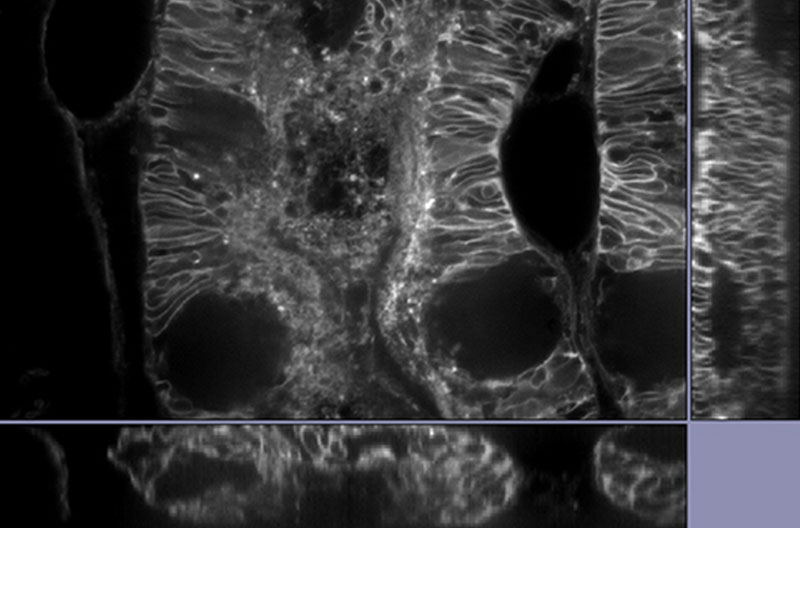
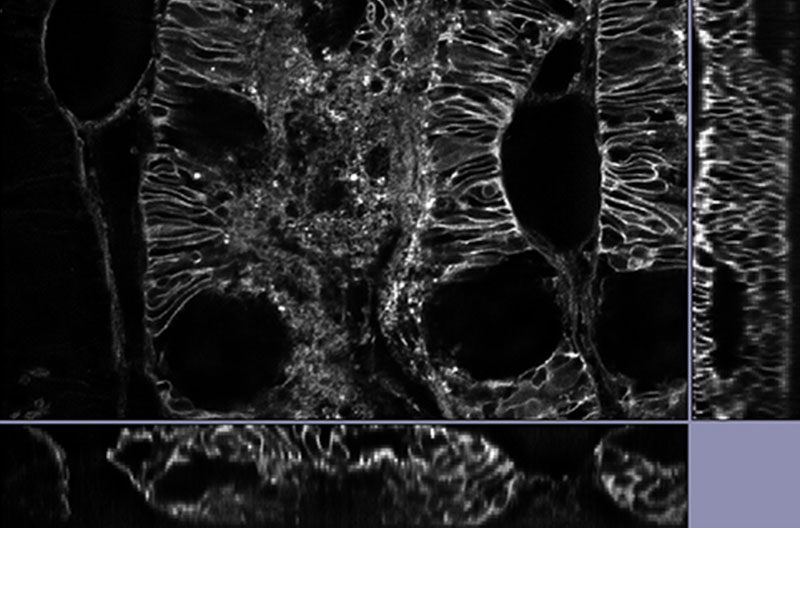
MULTI-MODALUsers can easily switch between 3 modes (widefield, confocal, and super resolution). *Image: Odf2 staining (Alexa 488) of cilia at the upper part of the basal body. Image courtesy of: Hatsuho Kanoh, Elisa Herawati, Sachiko Tsukita,Ph.D. Graduate School of Frontier Biosciences and Graduate School of Medicine, Osaka University. |
|---|
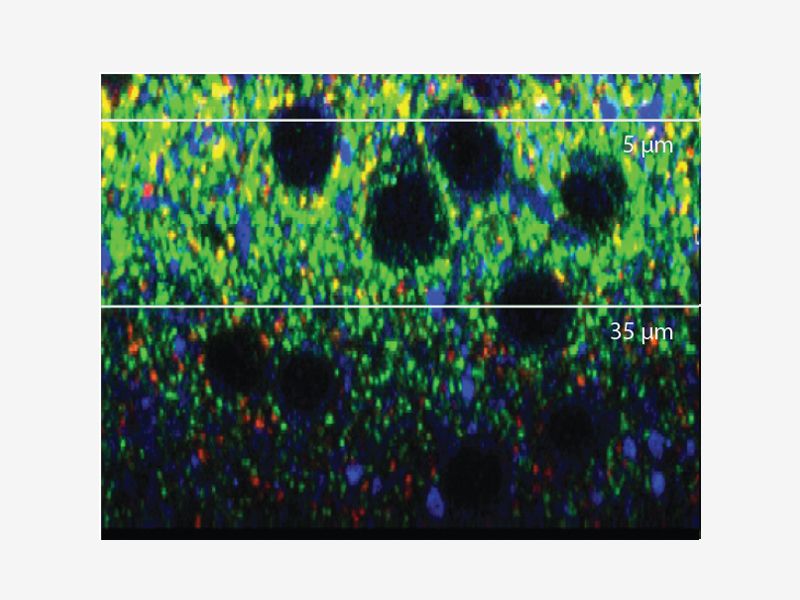
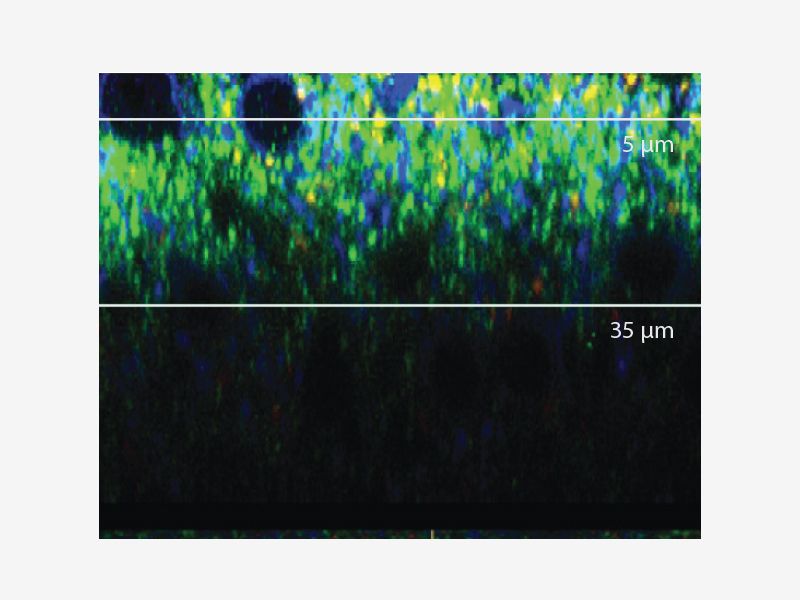
DEEP IMAGINGAccurate 3D reconstruction as the refraction index of silicone oil is close to that of your live sample medium. |
|---|
CLEAR IMAGEGet clear images using Olympus’ deconvolution algorithm. *Image: Mouse kidney tissue stained with Alexa488 |
|---|
OSR PRINCIPLEThrough improved detection, specific hardware settings and signal processing, Olympus has realized improved contrast with super resolution. The Olympus SpinSR technology realizes lateral (XY) resolution down to 120 nm. | 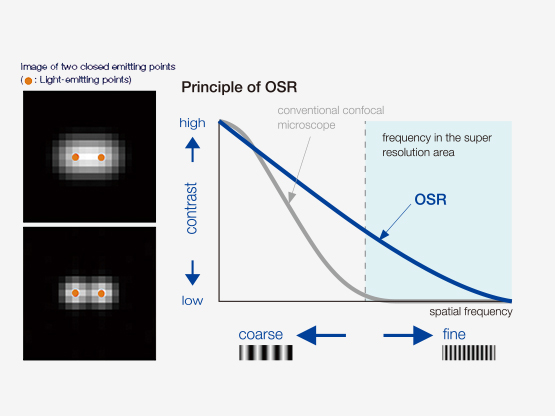 |
|---|
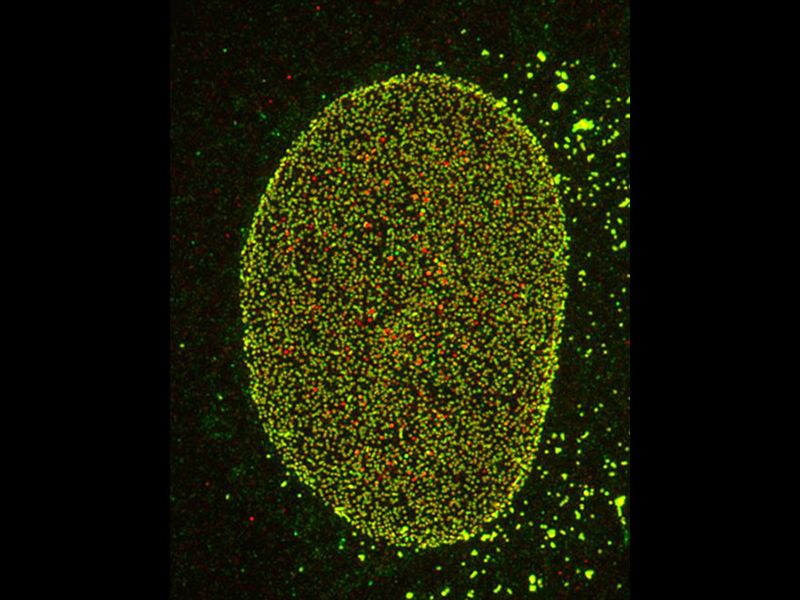
EASE OF USEGet multi-color imaging without using specific dyes. *Nuclear pore complex of Hela cell
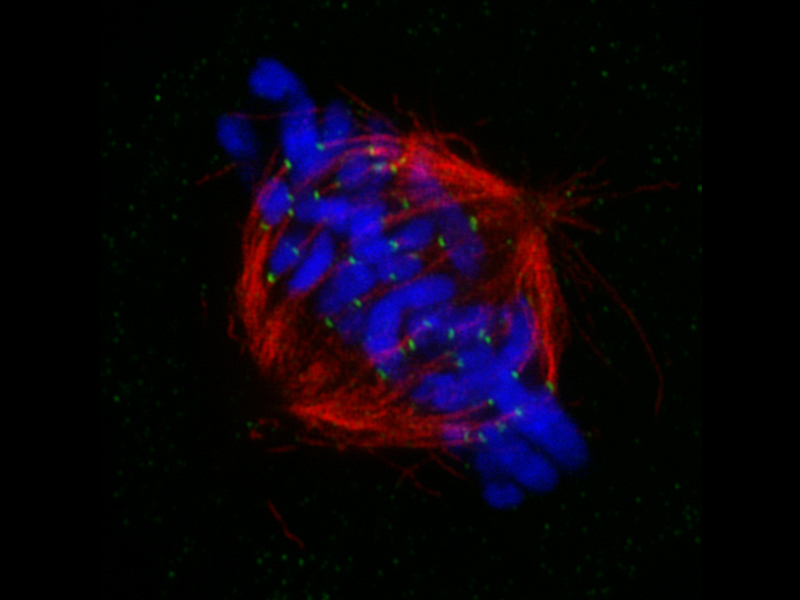
*Mitotic spindle at metapahse cell
|
|---|
Resources
Nothing matches your criteria.
References
S. Hayashi and Y. Okada, “Ultrafast superresolution fluorescence imaging with spinning disk confocal microscope optics,” Mol. Biol. Cell 26(9), 1743–1751 (2015).
S. Hayashi, “Resolution doubling using confocal microscopy via analogy with structured illumination microscopy,” Jpn. J. Appl. Phys. 55(8), 082501 (2016).
A. Nagasawa-Masuda and K. Terai, “Yap/Taz transcriptional activity is essential for vascular regression via Ctgf expression and actin polymerization,” PLoS ONE 12(4), e0174633 (2017).
H. Nakajima, et al., “Flow-Dependent Endothelial YAP Regulation Contributes to Vessel Maintenance,” Dev. Cell 40(6), 523-536.e6 (2017).
K. Tateishi, et al., “Three-dimensional Organization of Layered Apical Cytoskeletal Networks Associated with Mouse Airway Tissue Development,” Sci. Rep. 7, 43783 (2017).
E. Herawati, et al., “Multiciliated cell basal bodies align in stereotypical patterns coordinated by the apical cytoskeleton,” J. Cell Biol. 214(5) 571-586 (2016).
M.-T. Ke, et al., “Super-Resolution Mapping of Neuronal Circuitry With an Index-Optimized Clearing Agent,” Cell Rep. 14(11) 2718–2732 (2016).
Sorry, this page is not
available in your country.
Sorry, this page is not
available in your country.

.jpg?rev=A909)
.jpg?rev=3199)