Revolutionizing Confocal Microscopy
FLUOVIEW Smart™ Software Introduces Intuitive and AI-Enhanced Workflows
Confocal laser scanning microscopes have become indispensable tools in life sciences and medical research, enabling high-resolution observation and three-dimensional analysis of biological specimens such as cells and tissues. They are widely used in fields including cell biology, neuroscience, and developmental biology, serving as a foundational technology that supports research quality and reproducibility.
To meet diverse research needs, confocal microscopes have evolved to offer increasingly advanced features and greater flexibility in imaging conditions. However, this progress has also led to more complex software interfaces, creating significant challenges—especially for novice users—such as time-consuming setup, a higher risk of operational errors, and reduced efficiency in research workflows.
Challenges Posed by Complex Confocal Software Interfaces
- High Learning Curve: Mastering the operation requires considerable time, delaying the start of research. Beginners often struggle to know where to start, leading to frequent assistance from experienced users or instructors.
- Reduced Efficiency Due to Misoperation: With numerous settings and hard-to-find functions, workflows become fragmented. Errors can lead to data loss or sample damage, requiring costly re-imaging or re-experimentation.
- Issues in Shared Environments: When multiple users with varying experience levels share the same system, unnoticed changes to settings can result in unintended imaging conditions, wasted samples, and the need for repeat experiments.

Addressing these issues requires a software interface that is intuitive, streamlined, and designed around user workflows.
Optimizing the User Experience with FLUOVIEW Smart™ Confocal Software
Evident’s FLUOVIEW Smart confocal software is designed to help users of all skill levels operate the FLUOVIEW™ FV5000 laser scanning microscope both confidently and efficiently. It employs three key approaches to reduce operational burden and enhance research productivity and reproducibility.
Graphical UI and On-Screen Guidance to Lower Learning Costs
Basic microscope operations—such as Z-range settings and objective lens selection—are not merely configurations but essential information for understanding the current system state. FLUOVIEW Smart visualizes these settings graphically, allowing users to intuitively grasp their meaning and impact (Figures 1 and 2).
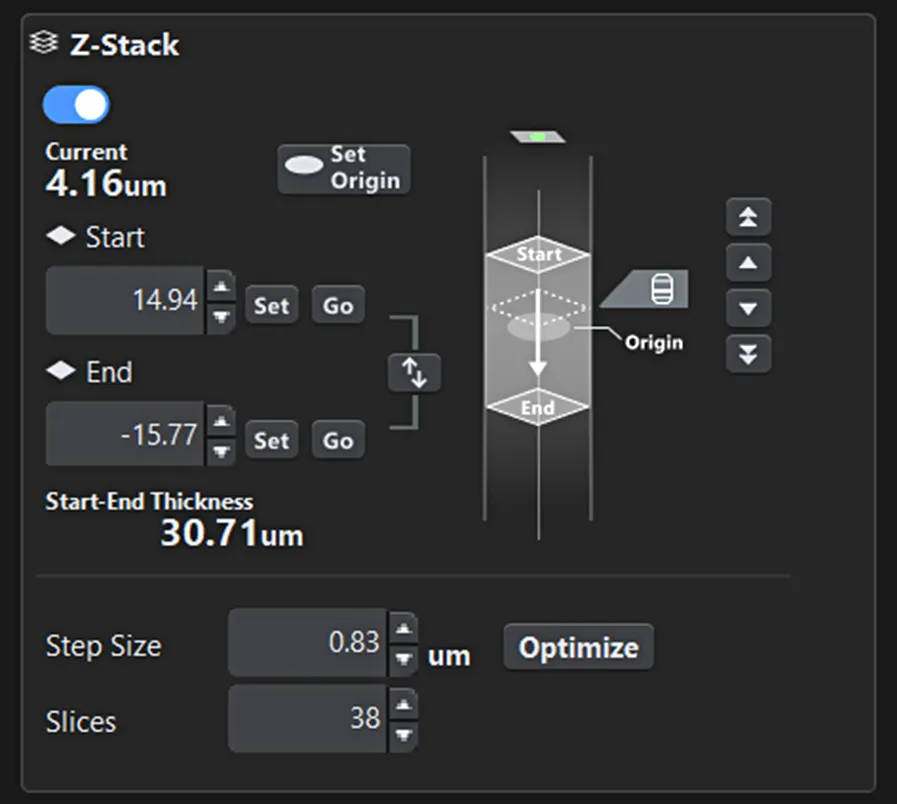
Figure 1. Z-range setting screen. The start and end positions for imaging, as well as the current observation position, are clearly visible at a glance.
Onscreen guides further assist users in performing tasks without confusion, reducing the need for constant supervision and enabling autonomous data acquisition.
Layout Based on Functional Priority
Instead of cramming all functions into a single screen, FLUOVIEW Smart™ software organizes them hierarchically by frequency and importance. Critical controls—such as laser power, scan range, and Z-stack settings—are always easily accessible, aligning with the natural workflow (sample detection → condition setup → imaging). This design minimizes search time and significantly reduces the risk of errors.
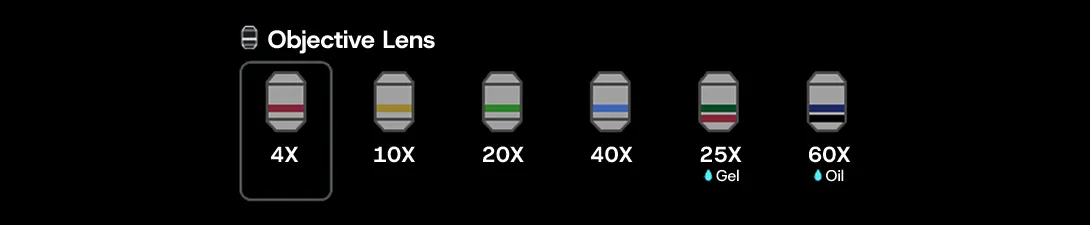
Figure 2. Objective lens selection screen. Users can intuitively select the desired objective lens, and the currently active lens is easily identifiable.
Reset and Purpose-Based Auto Layout
To prevent issues in shared environments, FLUOVIEW Smart software resets settings to default at startup. At the same time, it allows users to save and recall previous configurations for reproducibility. Additionally, selecting an imaging purpose (e.g., Z-stack, time-lapse, multiarea stitching) automatically adjusts the layout and guidance, providing an optimal interface for both beginners and experts (Figure 3).
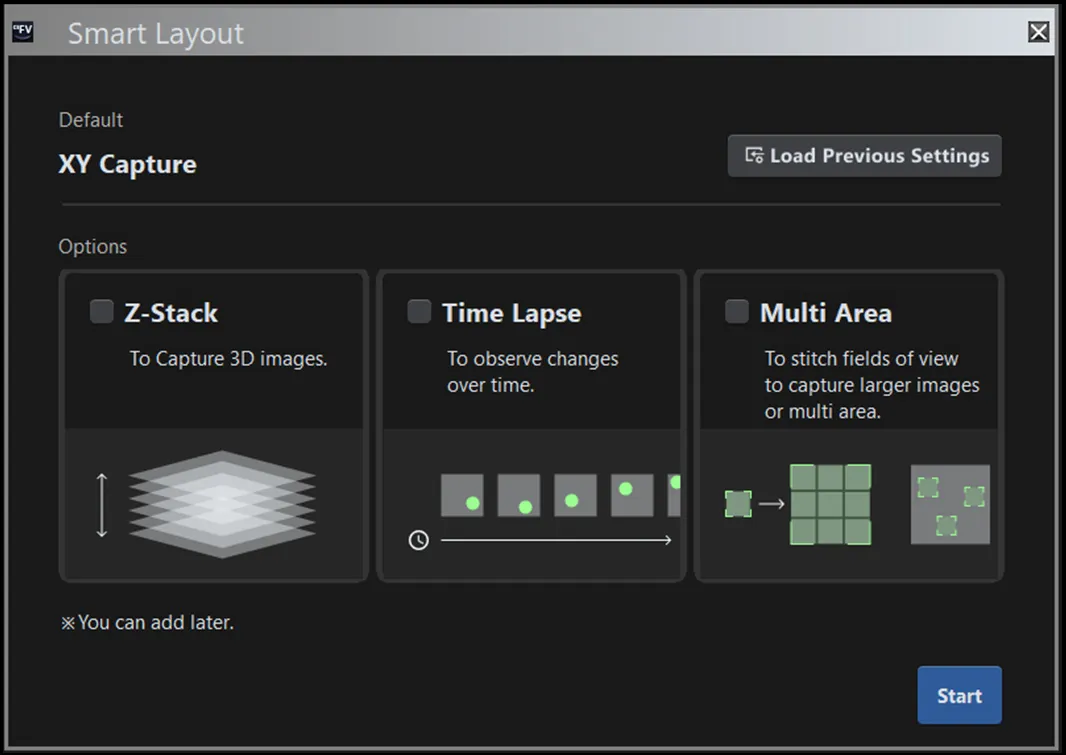
Figure 3. Purpose selection screen. The layout and onscreen guidance automatically adjust based on the selected objective.
AI-Powered Assistance for Novice Users
Beyond simplifying the interface, FLUOVIEW Smart™ software leverages AI to automate and optimize steps that are particularly challenging for beginners.
Automated Sample Detection
Due to the optical principles of confocal microscopy, if the sample is out of focus in either XY or Z, the screen may appear blank—leaving novice users uncertain whether the issue is due to settings or hardware malfunction.
To address this challenge, Evident and Epistra—leveraging their proven expertise in AI development for life sciences—jointly developed an image recognition algorithm that automatically switches the microscope to an optimal state for sample searching and identifies the sample region. This significantly simplifies and accelerates a process that was previously difficult for beginners (Figure 4).
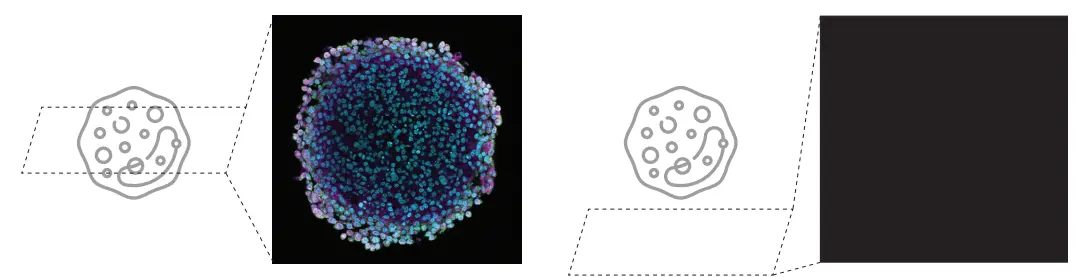
Figure 4. The high difficulty of sample searching in confocal laser scanning microscopy can lead to a blank screen (right image)
Figure 5 illustrates the overall workflow for sample position estimation.
The system is designed to mimic the expert user’s approach: performing coarse alignment of the Z and XY axes using a low-magnification objective lens (4X), followed by fine adjustments with a high-magnification lens (10X).
Specifically, the system executes the following four steps in sequence to automatically determine the optimal sample position.
Z-SEARCH (4X)
This step performs a sequential search* using a 4X objective lens to roughly identify the optimal Z-position within the search range. At each candidate Z-position, an image is captured, and the AI evaluates its focus score. Based on these results, the system autonomously updates the next Z-position to explore, iterating until the position with the highest focus score is reached. The Z-search range is appropriately set according to the user’s selection in the wizard.
XY-SEARCH (4X)
Using the image acquired with the 4X objective lens, the XY stage is moved so that the region with the highest local brightness is centered on the screen. Before this process, the system detects predefined artifacts (e.g., container edges). If artifacts are detected, an alert is displayed, and the process stops. The user can then manually adjust the sample’s XY-position as needed.
Z-SEARCH (10X)
After switching to a 10X objective lens, the system performs a sequential search to determine the optimal Z-position within the range. The Z-search range is determined based on the optimal Z-position found at 4X and the Z-resolution of the 4X lens.
XY-SEARCH (10X)
In the 10X image, the XY stage is moved so that the region with the highest local brightness is centered on the screen. After this process, the system displays the current microscope image to the user, completing the search procedure.
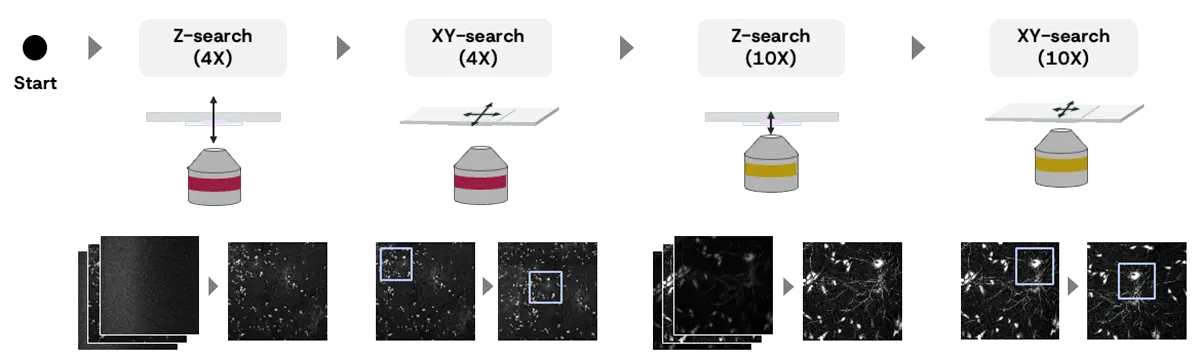
Figure 5. The Overall workflow for sample position estimation:
Z-SEARCH (4X)
- Roughly scan the specimen along the Z-axis with the confocal aperture open.
- Set the confocal aperture to 1 Airy unit and fine-tune the Z-position for optimal focus.
XY-SEARCH (4X)
- AI anomaly detection (if specimen is not detected or cover glass/well edge is detected).
- Move the stage to a brighter spot on the specimen.
- Reflect the 4X image on the map.
XY-SEARCH (4X)
- AI anomaly detection (if specimen is not detected or cover glass/well edge is detected).
- Move the stage to a brighter spot on the specimen.
- Reflect the 4X image on the map.
Z-SEARCH (10X)
- Roughly scan the specimen along the Z-axis with the confocal aperture open.
- Fine-tune the Z-position with 1 Airy unit confocal aperture for optimal focus.
XY-SEARCH (10X)
- Move the stage to a brighter spot on the specimen.
*Sequential search: a method that explores the sample position by capturing images sequentially across a defined search range.
Imaging Condition Optimization
In conventional confocal laser scanning microscopy, there is an inherent tradeoff between detector sensitivity and laser power, requiring trial and error to find the optimal settings. Beginners often face the dilemma that increasing sensitivity introduces more noise, while increasing laser power risks damaging the sample (Figure 6). As a result, determining the appropriate imaging conditions can be time-consuming. Evident addresses this challenge with its proprietary nextgeneration SilVIR™ detector technology, which eliminates the need to adjust detector sensitivity. This reduces the number of parameters users must configure, resulting in simpler operation. Consequently, setting the laser power to achieve the appropriate contrast becomes the most critical factor influencing image quality.
FLUOVIEW Smart™ software automatically determines the optimal laser power based on real-time image analysis and machine learning predictions. Users can easily apply settings tailored to their needs by selecting a mode according to the priority between minimizing sample damage and maximizing contrast.
TOO HIGH LASER POWERLaser power that is too high leads to high phototoxicity
Figure 6. Tradeoff between laser power and image quality. | TOO LOW LASER POWERLaser power that is too low leads to noisy images
|
The overall process is as follows:
MODE SELECTION
The user selects one of three modes—Gentle, Balanced, or Quality—based on the priority between sample protection and image contrast.
AUTOMATIC LASER POWER ADJUSTMENT
When the user clicks the execution button, the system automatically sets the optimal laser power and completes the process. During execution, the following steps occur internally:
- Four images are captured at different laser power levels.
- For each image, the system calculates an AI score as an indicator of image quality (steps 1–3 in Figure 7). The AI scores are plotted, and based on the graph and the selected mode, the system estimates the optimal laser power (step 4 in Figure 7).
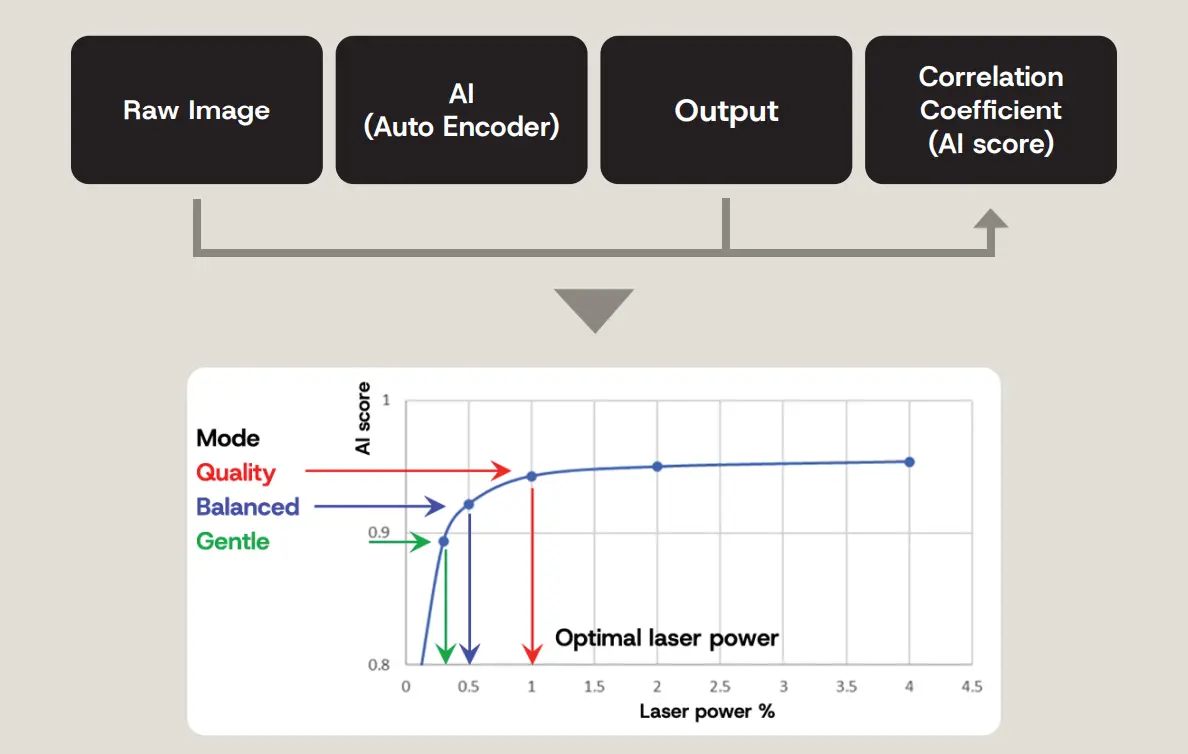
Figure 7. Concept behind laser power estimation with the following steps: 1: Predict clean, low-noise images from the raw image by using AI. 2: Compare the predicted image with the original raw image. 3. Calculate a correlation coefficient (AI score: high score is stable with low noise, low score is unstable with high noise). 4. Estimate the optimal laser power based on the selected mode.
Note: the AI score is similar to the image signal-to-noise ratio (SNR), though slightly different. The score increases with laser power, reflecting improved image quality due to reduced noise. The plateau region of the AI score curve indicates an appropriate laser power setting: in this range, further increases in laser power yield negligible improvements in image quality, making them inefficient.
Conclusion
FLUOVIEW Smart™ confocal software simplifies the interface for FLUOVIEW™ FV5000 laser scanning microscopes and enhances workflows through AI technology (Figure 8).
- Complex software interfaces pose a significant barrier for novice users.
- Simplification is achieved through prioritized functionality, workflow-based operation design, and a graphical UI.
- AI automates sample detection and laser power adjustment, greatly reducing operational complexity.
By combining a user experience–focused software interface with AI-driven smart assist features, FLUOVIEW Smart software enables even beginners to acquire high-quality images in a short time, dramatically improving productivity and reproducibility in research environments.
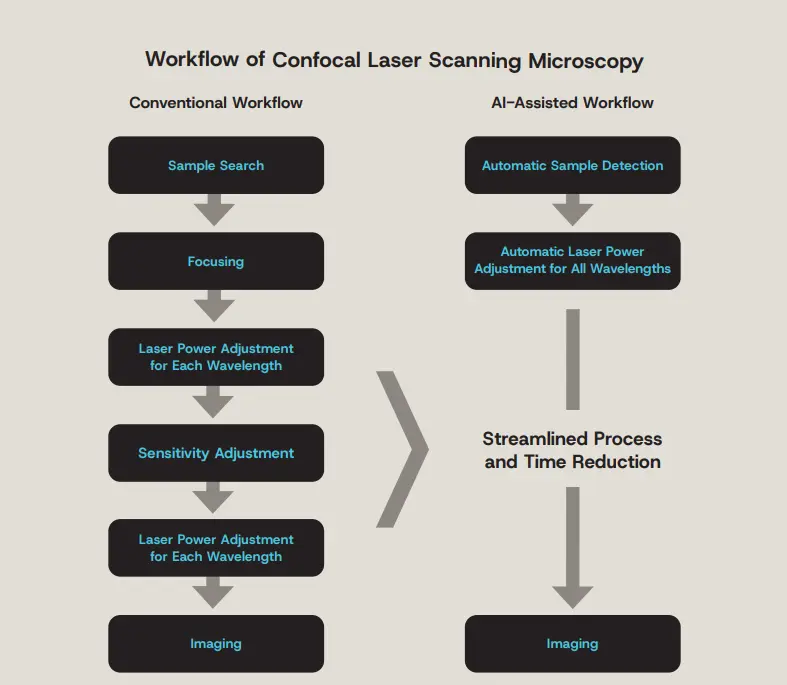
Figure 8. Streamlining confocal microscopy observation with an AI-assisted workflow.
Author
Ryoji Kitamura
Global Product Manager, High-End Imaging Systems, Life Science
Ryoji Kitamura earned his master’s degree from the Graduate School of Information Science and Technology at Hokkaido University, where he focused on in vivo imaging using multiphoton microscopy. He began his career at Evident as a software engineer and later became the product leader for the SLIDEVIEW™ VS200 universal whole slide imaging scanner. He also served as the Global Product Manager for the IXplore™ IX85 inverted microscope system. Currently, he is the Global Product Manager for the FLUOVIEW™ confocal microscope series, leading product strategy, planning, and development.
Products Related to This Application
was successfully added to your bookmarks
Maximum Compare Limit of 5 Items
Please adjust your selection to be no more than 5 items to compare at once
Not Available in Your Country
Sorry, this page is not
available in your country.